‘Immunology is obviously not perspicuous enough to eliminate these understandings, unless one maintains that arrogance blinds “scientific experts” from seeing obvious truths, which even a “plowboy” ought to be able to grasp.’ – adapted from Martin Luther, 1483-1546
A vaccine is a biological preparation that provides active acquired immunity to a particular infectious disease.
The various types of vaccines include:
- Inactivated
- Attenuated
- Toxoid
- Subunit
- Conjugate
- Heterotypic
- Synthesized Protein Epitope (mRNA, DNA, etc.)
It has been known for centuries that exposure to extremely small viral loads and/or attenuated or inactivated viruses can elicit an immune response without resulting in severe illness and create future immunity against serious infections. The Chinese practiced the oldest documented use of variolation, dating back to the fifteenth century. They implemented a method of “nasal insufflation” administered by blowing powdered smallpox material, usually scabs, up the nostrils. Other approaches included rubbing powdered smallpox scabs or fluid from pustules into superficial scratches made in the skin. Modern vaccines seek to minimize adverse effects while creating lasting immunity.
Vaccines for SARS-CoV-1 have been created and it is very likely that a vaccine for SARS-CoV-2 will become available very soon based on promising results from Phase 1 and Phase 2 studies. Update 12/2020: Phase 3 trials have completed and vaccine is available.
From vaccine development, we can learn that it is possible to elicit immune response from inactivated viruses as well as extremely small but viable viral loads. These centuries old understandings demonstrate that infection severity can be dependent upon viral load amount during exposure and that sufficiently small viral loads can create immunity with mild or possibly no perceived symptoms. An NIH study demonstrated that low viral load exposures caused people to develop increased immunity levels without becoming significantly sick – they “seroconverted while having minimal clinical illness and no shedding“ and in that study, there were 3 orders of magnitude range of viral load quantities that resulted in minor symptoms, while the 4th and 5th magnitude orders became significantly more severe. This means there was a 1000 fold range where minor illness was found, yet some level of immune response occurred.
In an anecdotal case which is not well documented but helpful for illustrating repeat exposure concepts, a nurse in New York during the COVID outbreak was serology tested for antibodies. The nurse was found to have a 1.2 signal-to-cutoff CLIA titre which is below the 1.4 S/C threshold for positive indication. He was tested again approximately 1 month later where the 2.4 S/C titre was much larger and clearly positive, The nurse claimed to be unaware of any symptoms throughout the period or recent past. One possible explanation is that the nurse had repeated low viral load exposures that circumvented PPE and that these repeated low viral load exposures resulted in an immune response such that antibody serology increased and ultimately was found at positive IgG levels and likely indicating immunity. that repeat exposure tends tends to result in both stronger and longer immunity.
In a study of 12541 health-care workers, 1265 (10%) tested positive for antibodies. Of those 1265 antibody positive individuals, 401 (32%) of them seroconverted without having recalled COVID-19 symptoms. They sercoconverted without memorable illness. It is likely this occurred as a result of low viral load exposure. “Of 1265 seropositive health care workers, 864 (68%) recalled having had symptoms consistent with those of coronavirus disease 2019 (Covid-19), including symptoms that preceded the widespread availability of PCR testing for SARS-CoV-2”
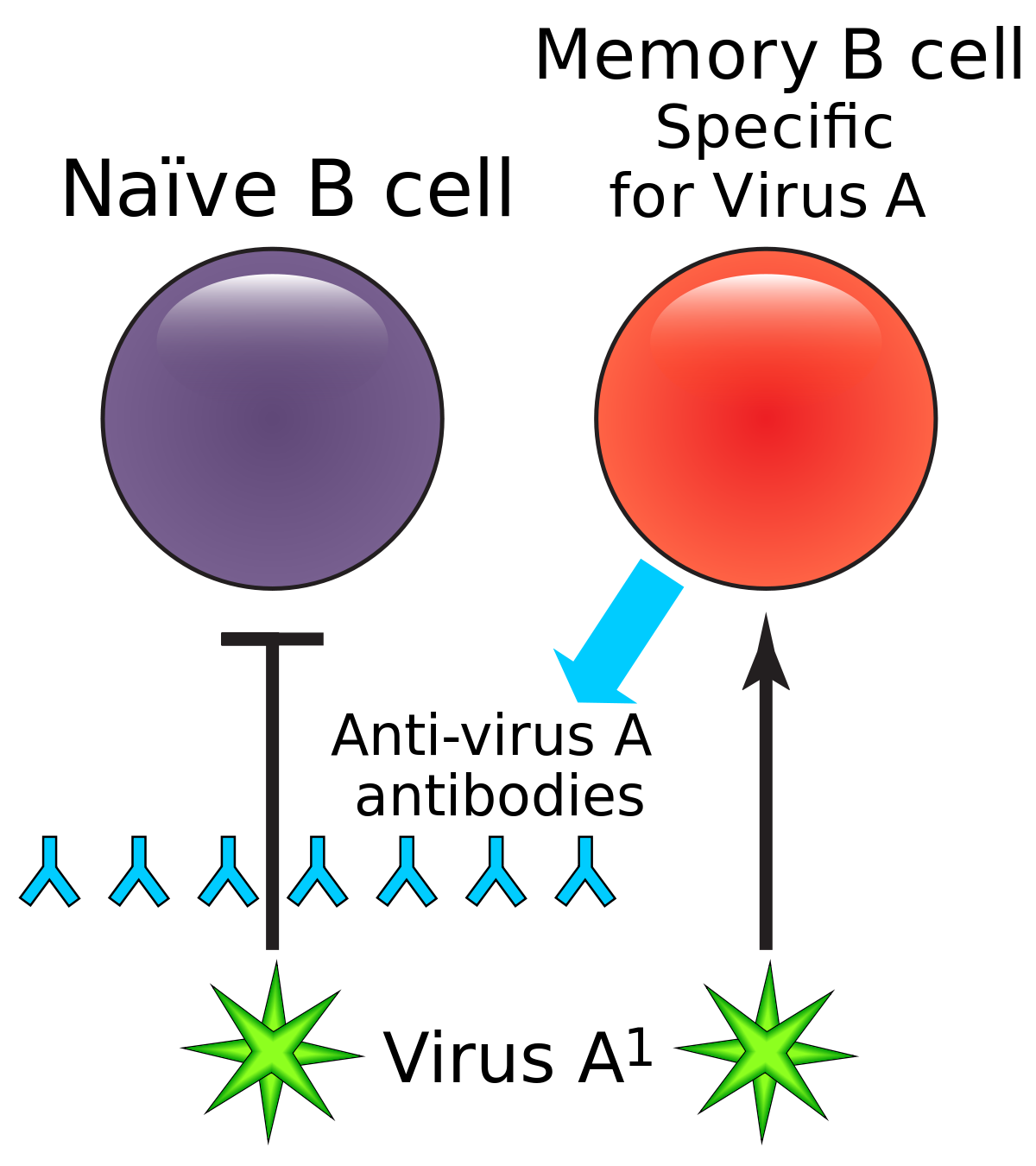
Some vaccines require a calendar schedule of 2 or more immunizations to create long-lasting immunity. While an initial dose can create short-term immunity, longer lasting immunity requires multiple exposures to the vaccine. From this, it can be deduced that exposure to viable viruses may in some cases have similar responses. Long-lasting immunity may not occur on first exposure with some viruses. While it seems counter-intuitive, it may be beneficial for someone who has recovered from an infection to remain in an environment where infections are continually occurring for some time period so that, even weeks or months after recovering, the individual may re-encounter the virus and re-engage an immune response. In these cases, the person, having some remaining antibodies (and perhaps more important, some B memory / plasma cells) still present in their blood will rapidly defeat the virus and likely be asymptomatic having no perceived illness, yet the immune system has responded and also rebuilds and replicates additional antibodies and related Tmem, CD4, CD8, and broadly, other leukocytes. Additional exposures to a pathogen after having recovered from an initial infection most often strengthens and lengthens the persons immunity to that pathogen. Avoiding multiple exposures to a virus through isolation measures has potential to have a detrimental effect on long-lasting immunity.
Cross Reactivity
In some cases, having immunity to one virus can result in a reaction to a different virus. This is known as cross-reactivity, cross-immunity, or cross-protective immunity. In rare cases, cross reactivity can result in complications. In the case where cross-reactivity results in cross-immunity, it may result in an asymptomatic infection such that there is no perceived illness. Since the cross-reactive immunity from an older virus largely defeats the new virus, the individual would likely be asymptomatic, perceive no illness, and would likely shed little or no virus that could infect others. The possibility of developing a direct immune response and direct immunity to the new virus also exists as some fraction of the new viral load may result in B and T cell / plasma development that is specific to the new virus as noted above. Regarding COVID and cross-reactivity, “A study of blood samples taken before the COVID-19 pandemic showed that some people already had certain immune cells that recognize SARS-CoV-2. These immune cells also reacted with coronaviruses that cause common colds.”. “T-cell studies are also converging on the possibility of cross-reactivity, in which T cells that recognize other coronaviruses also recognize SARS-CoV-2.” Another article stated “At least six studies have reported T cell reactivity against SARS-CoV-2 in 20% to 50% of people with no known exposure to the virus”. A recent study suggests that cross-reactivity in those who serconverted from exposure to SARS-CoV-1 could confer some level immunity to SARS-CoV-2. It is possible that SARS-CoV-1 was more widespread than understood and now protective of those who were exposed, possibly being causal of lower SARS-CoV-2 cases in Asia. Given that immunity is an analog rather than binary characteristic, it also would help explain asymptomatic COVID infections in Asia. Subclinical (unobserved) infections from SARS-CoV-1 appear to have been quite prevalent in Asia according to Seroprevalence of IgG antibodies to SARS-coronavirus in asymptomatic or subclinical population groups. Another study appeared to show cross reactive antibodies present in blood samples taken between 2011 and 2019.
Outbreak Natural Immunization Potentials
The 1000 fold range mentioned above is an easily hit target in some natural settings – a breezy place could be a natural random chance vaccination factory that exposes one to only a few virions – perhaps within a much narrower range. Repetitive low level exposure could further strengthen immune response. Perhaps a 15th century Chinese elder refining a variolation process is not needed. And perhaps it may be possible to access some life-saving individual and herd-immunity more rapidly than a modern vaccine that must be synthesized and then human trialed for safety and efficacy. While waiting for vaccine development and limiting exposure to variolative ranges, individuals at low-risk for a severe case could gain immunity without becoming sick while enjoying normal outdoors activity. Similarly, wearing masks indoors may also put exposure viral load into the variolation range when kept within limited time durations. And it is certainly important to be conscientious to protect others in essential marketplaces using distance and other Safety Protocols to minimize infection probability of a vector [person] that then might bring the virus to a very high-risk for severe case individual or health care setting. Those in direct contact with the high-risk may need to forgo riskier settings during outbreaks.
COVID-19 Immunity
Many popular news stories have questioned whether someone who has recovered from COVID-19 has any immunity, claiming that antibody titers decreased within a few months and that little of what we do know about SARS, MERS, or immunology in general applies to SARS-CoV-2. While there is always much to learn, there are many counter-arguments to this claim. It is important to note that IgG antibody titers generally peak during infection and generally subside dramatically several weeks after the infection resolves for virtually all types of viral infections.
1) SARS-CoV-2 antibody serology data taken around the world demonstrates that people do develop antibodies that persist for significant periods of time. Large numbers of people have been found to have measurable IgG antibodies several months after infection.
2) On the order of tens of millions of people in the US have recovered from COVID-19 and the if the symptomatic re-infection percentage was large there would be a large concern – instead the documented cases of reinfection appear to be the rarity and/or asymptomatic rather than the rule and the media has been rapid to report these cases via stories of anecdotal cases. That said, for any viral infection, it is not completely unusual for secondary infections to occur as they are most often milder and serve to strengthen future immune response. While there may be a small statistical cluster of symptomatic / increasing symptom re-infection cases, it is not the large majority of cases.
3) Two vaccines had positive results in rhesus macaques: “mRNA-1273 protected against a high dose SARS-CoV-2 infection in non-human primates and prevented pulmonary disease in all animals” and ““The virus was cleared very rapidly in the vaccinated animals,” said Barney S. Graham, senior author of the study and deputy director of the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases.”.
Study excerpt: “At day 7, in animals vaccinated with 10 μg of mRNA-1273, inflammation was mild, and no viral RNA was detected (Figure 4A). However, at day 8, one animal in the 10-μg dose group had a single pneumocyte that was positive for viral antigen (Fig. S8). No substantial inflammation was observed in the lungs of nonhuman primates vaccinated with 100 μg of mRNA-1273, and neither viral RNA nor antigen was detected at day 7 or 8 after challenge (Figure 4A). In addition to the lung sections from these earlier time points, lung sections from animals that were killed at day 14 or 15 after challenge had no evidence of substantial inflammation, and neither viral RNA nor viral antigen was detected in any of the groups, including the control group“.
Also see: COVID-19 Vaccine Study Data.
4) A SARS-CoV-2 live virus primary and re-exposure study on rhesus macaques appears to align with the current observations of human re-infections and suggests “that primary SARS-CoV-2 exposure protects against subsequent reinfection in rhesus macaques.“. Another similar study stated “SARS-CoV-2 infection in rhesus macaques led to humoral and cellular immune responses (Fig. 2) and provided protection against rechallenge“. A third study showed similar results. Human re-infection challenge trials are being debated in ethics circles.
5) More importantly, it is established general immunology, which COVID-19 cannot escape, that B memory cell prevalence is what maintains long-term immunity. There is not always a strong correlation between B memory cell prevalence and IgG antibody prevalence. It is normal and necessary for the body to eliminate antibodies over time. In fact, a bioscience Director of Research once quipped “How would our immune system look if we were churning out massive amounts of antibodies to everything we ever encountered? We’d be a giant lymph node.”
Homeostatic processes maintain T and B memory cell populations. B cells appear to survive for long periods due to anti-apoptosis mechanisms. Antibodies, not being living cells, do not replicate / divide – they are produced by B cells. IgG antibodies tend to have half-lives on the order of 20 days. When B cells encounter an antigen, the ones with receptors that bind with a certain affinity begin to proliferate and mature, first secreting IgM then undergoing a class shift and maturation to plasma cells that secrete IgG and IgA depending various factors. Studies of a large set of different viruses have shown that “memory B-cell numbers did not correlate with antibody titers“. This means that having a low antibody count does not necessarily imply low B memory cell count or lack of long-term immunity; although it may be indicative, it also may not be indicative at all. B-cell prevalence is the best indicator of long-term immunity as B cells are the ‘factories’ that create antibodies.
6) T cell mediated immunity. T cells that provide immunity were found in a study, regardless of antibody titer levels. T cells can activate B cells and cause secretion of antibodies. Importantly, SARS-CoV-2-specific T cells were detectable in antibody-seronegative exposed family members and convalescent individuals with a history of asymptomatic and mild COVID-19. Our collective dataset shows that SARS-CoV-2 elicits robust, broad and highly functional memory T cell responses, suggesting that natural exposure or infection may prevent recurrent episodes of severe COVID-19.
7) A comprehensive study stated: “we found that individuals that recovered from mildly symptomatic COVID-19 had an expanded arsenal of SARS-CoV-2-specific immune mediators: neutralizing antibodies, IgG+T-betlo classical MBCs, circulating cytokine-producing CXCR5+ Tfh1 cells, proliferating CXCR3+ CD4+ memory cells and IFNγ producing CD8+ T cells that were maintained to at least three months post-symptom onset. This study predicts that these recovered individuals will be protected from a second SARS-CoV-2 infection and, if so, suggests that Th1 memory should be the target of vaccine elicited memory“.
8) Direct secondary exposure and immunity of humans was documented on a fishing expedition where many people became infected during the expedition, but those who had antibodies from a prior infection were protected. “Predeparture serological and viral RT-PCR testing along with repeat testing after return to shore was available for 120 of the 122 persons on board over a median follow-up of 32.5 days (range 18.8 to 50.5 days).” and it was found that “the presence of neutralizing antibodies from prior infection was significantly associated with protection against re-infection”.
9) A popular news media story “How worrying are reports the first person in the world has been re-infected with coronavirus?” seems to have a worrisome title implying that it may be necessary to “worry” about being “re-infected” and that immunity does not exist. However, when looking into the details of the story and the scientific study that led to the story, it can be concluded that the study documents that the patient did not experience symptoms and otherwise demonstrated one of the longest periods of immunity to date. When re-challenged by the virus 4.5 months later, the patient was asymptomatic and did not present any signs of illness. In fact, the detailed scientific study indicated that no IgG antibodies were measurable at day 1, but they were present on or before day 5 which implies that the patent was protected by B / T cell mediated responses and then began developing measurable IgG antibodies within a few days. The study also indicated that viral loads decreased during the period the patient was observed, implying that viral load shedding was minimal and that the patient was not rapidly replicating the virus – which would mean the patient might have been slightly infectious, but not strongly so, and became consistently less infectious during the period of observation. Additionally the second exposure re-challenge was from a slightly mutated SARS-CoV-2 viral genome that was different from the first primary infection the patient experienced, yet the patient did not become ill during the second exposure and appeared to be protected by B/T cell mediated immunity that rapidly secreted IgG from existing SARS-CoV-2 specific B cells. This study may also be showing the negative impact of excessive isolation measures on the rapid immunity of those who have recovered, as it took a few days to rebuild IgG antibody levels, creating a short window of being somewhat infectious to others. Somehow, the good news of a scientifically documented case of someone appearing to exhibit immunity after 4.5 months, turned into a “worrisome” news headline.
10) Viewpoint article from JAMA on achieving SARS-CoV-2 immunity: COVID-19 and the Path to Immunity
11) A Science Immunology article entitled Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients measured “plasma and/or serum antibody responses to the receptor-binding domain (RBD) of the spike (S) protein of SARS-CoV-2 in 343 North American patients infected with SARS-CoV-2 (of which 93% required hospitalization) up to 122 days after symptom onset”. A similar article had additional information Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients.
12) Neutralizing and spike-specific antibody production persists for at least 5-7 months
13) Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. “An antibody first identified in a blood sample from a patient who recovered from Severe Acute Respiratory Syndrome in 2003 inhibits related coronaviruses, including the cause of COVID-19“
14) Robust SARS-CoV-2-specific T-cell immunity is maintained at 6 months following primary infection
15) Immunological memory to SARS-CoV-2 assessed for greater than six months after infection which is now published as Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection
16) Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. “As we have shown that SARS-CoV-2-specific Bmem cell numbers are stable over time, we propose that these Bmem may represent a more robust marker of long-lived humoral immune responses than serum antibodies… our results indicate that SARS-CoV-2 infection generates long-lasting B cell memory up to 8 months post-infection that could be protective against systemic disease upon reinfection.” And it’s likely to be much more than 8 months as that was a time limitation of the study.
17) Antibody Responses 8 Months after Asymptomatic or Mild SARS-CoV-2 Infection: “We analyzed data from 7 participants with asymptomatic SARS-CoV-2 infection and 51 patients with mildly symptomatic COVID-19 (Table 1). Eight months after their infections, we detected anti-N pan-Ig in 53 (91.4%), anti-N IgG in 15 (25.9%), anti-S IgG in 50 (86.2%), and anti-S1 IgG in 40 (69.0%) (p<0.01) (Table 2). The sVNT found positive neutralizing activity for 31 (53.4%).”
18) Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers: “The presence of anti-spike or anti-nucleocapsid IgG antibodies was associated with a substantially reduced risk of SARS-CoV-2 reinfection in the ensuing 6 months”
19) The humoral memory response in a cohort of 87 individuals assessed at 1.3 and 6.2 months after infection with SARS-CoV-2. We find that titres of IgM and IgG antibodies against the receptor-binding domain (RBD) of the spike protein of SARS-CoV-2 decrease significantly over this time period, with IgA being less affected. Concurrently, neutralizing activity in plasma decreases by fivefold in pseudotype virus assays. By contrast, the number of RBD-specific memory B cells remains unchanged at 6.2 months after infection. Memory B cells display clonal turnover after 6.2 months, and the antibodies that they express have greater somatic hypermutation, resistance to RBD mutations and increased potency, indicative of continued evolution of the humoral response.
20) Here we show that of the 32 individuals tested that were born in or before 1915, each showed seroreactivity with the 1918 virus, nearly 90 years after the pandemic. We isolated B cells from subjects and generated five monoclonal antibodies that showed potent neutralizing activity against 1918 virus from three separate donors. These antibodies also cross-reacted with the genetically similar HA of a 1930 swine H1N1 influenza strain
Immunity Summary
While it is possible that SARS-CoV-2 immunity might be short-lived, Dr. Faiqa Cheema, an HHC infectious disease specialist, stated that most people who get infected with COVID-19 will have an immune response. Some responses will be better than others. “It can be assumed safely that this immune response will offer some level of protection, at least for this year,” she said. “At this time, we do not know how long this immunity will last.”. Many sources including Dr. Fauci are expecting an approved vaccine before that year elapses. Again, knowing that B memory cells are what produce antibodies, a lack of antibodies does not confirm lack of immunity. Presence of T cells also indicate immunity. It is also known that repeat exposure tends tends to result in both stronger and longer immunity. “Repeat exposure has the effect of refining IgG affinity of the antibodies and boosting the number of memory cells”.
A Journal Of Immunology article stated: “the normal cadence by which we discuss science with our colleagues failed to properly convey likelihoods of the immune response to SARS-CoV-2 to the public and the media. As a result, biologically implausible outcomes were given equal weight as the principles set by decades of viral immunology. Unsurprisingly, questionable results and alarmist news media articles have filled the void. We suggest an emphasis on setting expectations based on prior findings while avoiding the overused approach of assuming nothing. After reviewing Ab-mediated immunity after coronavirus and other acute viral infections, we posit that, with few exceptions, the development of protective humoral immunity of more than a year is the norm. Immunity to SARS-CoV-2 is likely to follow the same pattern.”
It is likely that B memory cells related to COVID-19 would persist for many years, possibly decades. It is expected that during those years, the SARS-CoV-2 virus will mutate sufficiently to partially evade the immune response and protection will gradually diminish over time; although, subsequent exposure to the mutating virus over those years will build additional B memory diversity that will track those viral mutations. It is also likely that wide exposure / recovery during the pandemic and subsequent vaccination will dramatically reduce mutations as total viral replications subside. What’s most likely to occur, given general immunology understandings, is that, as the pandemic winds down, people who were vaccinated-against / recovered-from the early circulating 2019 virus will encounter a variant that developed during the pandemic peaks and develop an asymptomatic / minor infection, develop additional B / T / antibody diversity that covers the variant while the original B / T / antibody diversity largely clears the minor infection, and be then be protected against variants for many years to come as mutation / variant production subsides due to dramatically lower mutation rates as a result of the herd-immunity gained during the pandemic and 2021 vaccination efforts. It is unlikely that a continuing set of variant waves will cause a continuing set of pandemics, although it is likely that variant vaccines will continue to be marketed along with flu vaccines. We now have direct evidence of COVID-19 related long-lived plasma cells (LLPC) that suggest immunity will last for many years / decades.
Virus Lab Experiments
If this virus did indeed escape a lab, it is possible that future escapes will occur. While there is absolutely no desire for anyone to pander to conspiracy theories, it is known that gain of function lab experiments intended to develop understanding of how viruses mutate over decades and centuries and move to humans from animal reservoirs do occur. For example, PREDICT operated on five-year funding cycles,[5] receiving about $200 million over the course of its decade in operation. While it is not publicly known exactly what experiments were executing during those 10 years, there are known possible experiments that could occur. For example, an experimental process could accelerate mutation through scientifically engineered selection processes. A zoonotic virus that does not normally infect humans can be experimentally exposed to human cells under conditions which maximize success of infection and any resulting viral infection is then harvested for the next generation of exposure. This process is rapidly repeated until the virus becomes adept at infecting human cells. This process is known as serial passage. More direct genetic engineering methods are also available and utilized. The process can create something that would naturally take 10s to 1000s of years to occur naturally. In fact, it may never occur naturally, because the human immune system would adapt incrementally “each year” and prevent this kind of large gap mutation from ever occurring without a corresponding development of immunity via the polyclonal immune response that accompanies infections and then halts or severely limits the mutation process going forward. If the lab experiment escapes, the virus with a large unnatural mutation gap will then interact with human immune systems and likely result in a pandemic which the experiment was seeking to predict rather than cause. But again, in nature, the human immune response would develop alongside the viral mutation, preventing the large gap. Only a naive scientist (or one curious beyond reason) would not understand that this kind of experiment does not have any real predictive ability, although it does have the ability to create dangerous viral mutations.
As a proponent of virus research in China, Dr. Anthony Fauci stated in a October 9, 2012 article: “Putting aside the specter of bioterrorism for the moment, consider this hypothetical scenario: an important gain-of-function experiment involving a virus with serious pandemic potential is performed in a well-regulated, world-class laboratory by experienced investigators, but the information from the experiment is then used by another scientist who does not have the same training and facilities and is not subject to the same regulations. In an unlikely but conceivable turn of events, what if that scientist becomes infected with the virus, which leads to an outbreak and ultimately triggers a pandemic? Many ask reasonable questions: given the possibility of such a scenario—however remote—should the initial experiments have been performed and/or published in the first place, and what were the processes involved in this decision? Scientists working in this field might say—as indeed I have said—that the benefits of such experiments and the resulting knowledge outweigh the risks“.
Two months before the novel coronavirus is thought to have begun its deadly advance in Wuhan, China, the Trump administration ended a $200-million pandemic early-warning program aimed at training scientists in China and other countries to detect and respond to such a threat. Interestingly, On 19 December 2017 under the Trump administration, the NIH lifted the Obama moratorium into GoFR. As one might imagine, the research could be “marketed” as beneficial and an inappropriate shutdown as part of partisan politics. It can only be guessed what was really happening “under the hood” by those who desired GoFR funding, satisfying intellectual curiosities, etc. One point of clarity is that both Obama and Trump administrations placed moratoriums on Gain of Function research but it is not clear why GoFR funding had to be halted twice, first by Obama, and then again by Trump.
A zoonotic disease research program was launched after an H5N1 bird flu outbreak, Predict was part of an effort to search for previously undiscovered zoonotic diseases, which are passed from animals to humans. Viruses such as AIDS, SARS, MERS, Ebola, and certain influenza strains originally came from animals. Researchers found more than 1,000 new viruses from animal samples collected during the program’s run, including a new Ebola strain. A Lancet paper suggests that new programs should replace it: “As new funding opportunities pick up where PREDICT left off, making this work a priority will help move the field towards risk assessment that supports targeted prevention efforts” Interestingly, in 2018, an article “makes the case against performing exceptionally dangerous gain-of-function experiments.”. It concludes that: “the unique scientific and public health value of [potential pandemic pathogens] PPP experiments is inadequate to justify the unique risks they entail and that researchers would be well-advised to turn their talents to other methodologies that will be safe and more rewarding scientifically.”
Interestingly, it appears that just before the start of the pandemic, the WIV was using GoF experiments with chimeras in an attempt to create a vaccine. These experiments appeared to have included infecting mice genetically modified to express the human ACE2 protein with these chimeras. While it’s very speculative to identify the origin of SARS-CoV-2, it is clear that gain of function experiments are dangerous. Many pandemics appear to have zoonotic origins. “The National Institutes of Health earmarked $600,000 for the Wuhan Institute of Virology over a five-year period to study whether bat coronaviruses could be transmitted to humans, White House chief medical adviser Dr. Anthony Fauci told lawmakers Tuesday.” 5/25/2021
Construction of bat SARS-CoV pathogens for experiments on human ACE2 cells are documented to have occurred; for example: Using the reverse genetics technique we previously developed for WIV1 [23], we constructed a group of infectious bacterial artificial chromosome (BAC) clones with the backbone of WIV1 and variants of S genes from 8 different bat SARSr-CoVs. Only the infectious clones for Rs4231 and Rs7327 led to cytopathic effects in Vero E6 cells after transfection (S7 Fig). The other six strains with deletions in the RBD region, Rf4075, Rs4081, Rs4085, Rs4235, As6526 and Rp3 (S1 Fig) failed to be rescued, as no cytopathic effects was observed and viral replication cannot be detected by immunofluorescence assay in Vero E6 cells (S7 Fig). In contrast, when Vero E6 cells were respectively infected with the two successfully rescued chimeric SARSr-CoVs, WIV1-Rs4231S and WIV1-Rs7327S, and the newly isolated Rs4874, efficient virus replication was detected in all infections (Fig 7). To assess whether the three novel SARSr-CoVs can use human ACE2 as a cellular entry receptor, we conducted virus infectivity studies using HeLa cells with or without the expression of human ACE2. All viruses replicated efficiently in the human ACE2-expressing cells. The results were further confirmed by quantification of viral RNA using real-time RT-PCR. This work was jointly funded by National Natural Science Foundation of China (81290341, 31621061) to ZLS, China Mega-Project for Infectious Disease (2014ZX10004001-003) to ZLS, Scientific and technological basis special project (2013FY113500) to YZZ and ZLS from the Ministry of Science and Technology of China, the Strategic Priority Research Program of the Chinese Academy of Sciences (XDPB0301) to ZLS, the National Institutes of Health (NIAID R01AI110964), the USAID Emerging Pandemic Threats (EPT) PREDICT program to PD and ZLS, CAS Pioneer Hundred Talents Program to JC, NRF-CRP grant (NRF-CRP10-2012-05) to LFW and WIV “One-Three-Five” Strategic Program (WIV-135-TP1) to JC and ZLS.
One study demonstrated synthetic construction of a new coronavirus: Both wild-type and chimeric WIV-CoV infectious clones were designed using published sequences and based on the SARS-CoV infectious clone (10). Synthetic construction of chimeric mutant and full-length WIV1-CoV were approved by the UNC Institutional Biosafety Committee and the Dual Use Research of Concern Committee.
Some other NIH studies stated: “we synthesized the SHC014 spike in the context of the replication-competent, mouse-adapted SARS-CoV backbone (we hereafter refer to the chimeric CoV as SHC014-MA15) to maximize the opportunity for pathogenesis and vaccine studies in mice” and another: “In order to continue working with unaltered human clinical isolates of SARS-CoV, mouse strains constitutively expressing the human receptor for SARS-CoV, human angiotensin converting enzyme 2 (hACE2), were generated.“ One could interpret this to mean that mice were genetically altered to have human genomic content via chimeric processes so that they have a humanized ACE2 (where ACE2 facilitates viral entry to a cell) and that a synthetic/altered virus spike was created on an existing SARS-CoV virus and the mice were infected with it. When these are put together, a virus with a synthetic spike engineered to infect the humanized mice is available and replicated inside infected mice. Add a little time and selective pressure and the virus would be ready for the NIAID/Moderna team to test the mRNA vaccine they had been co-developing. Or something like that. Whatever these experiments were that went further, DARPA chose not to fund them stating that they entailed to much risk of leading to a pandemic.
“Darpa proposals, leaked to the pandemic origins analysis group Drastic, show the team had planned to take sequences from naturally occurring coronaviruses and use them to create a brand new sequence that was an average of all the strains. The grant application, submitted in 2018, states: “We will compile sequence/RNAseq data from a panel of closely related strains and compare full length genomes, scanning for unique SNPs representing sequencing errors. “Consensus candidate genomes will be synthesised commercially using established techniques and genome-length RNA and electroporation to recover recombinant viruses.””
“DRASTIC was recently made aware of documents provided by a whistleblower, which show that EcoHealth Alliance (EHA) in concert wIth the Wuhan Institute of Virology (WIV) attempted to carry out advanced and dangerous human pathogenicity Bat Coronavirus research that would clearly qualify as Gain of Function (GoF), in a grant proposal submitted to the Defense Advanced Research Projects Agency (DARPA) in 2018.”
“GoF experiments have neither prevented a pandemic, nor provided useful information about safe and effective pandemic countermeasures. Numerous prominent scientists argue that these experiments deviate from morally justifiable research, and the experimentally altered pathogens have put the entire human species at risk”. – Alliance for Human Research Protection (AHRP which has about 40 distinguished doctors on it’s advisory board)
While it may be helpful to surveil, observe, and take action against bats and other animal viral reservoirs to minimize zoonotic pathogen jumps to humans, it is not helpful and very dangerous to perform experiments that optimize conditions such that human cell infection occurs in the lab and produces viral progeny genetically engineered and/or selected for human cell infection.
Currently, it is indeterminate as to whether the COVID-19 pandemic was caused Virus Lab Experiments; but it is clear that these kind of experiments are much more likely to cause pandemics than they are to prevent them. These kind of experiments should not be done.
Synthetic construction of chimeric mutant and full-length SHC014-CoV has occurred in labs, resulting in significant epitope changes from the natural virus from which a novel coronavirus was derived. Large epitope changes render prior immune response to the natural virus much less effective and create new and large challenges to the adaptive immune system. The unique epitopes of SARS-CoV-2 suggest that it is a result of laboratory process, not a natural variant of a naturally occurring virus. As can be seen in the tutorial below, immune responses from natural infection and vaccines generate a polyclonal B/T/antibody response that is largely protective against variants. Only large variation would likely create such a novel threat such that existing antibody/B/T responses yield little/no protection. The rapid rate at which SARS-CoV-2 spread throughout the world suggests that it’s highly infectious epitopes were a result of an efficiency improving lab process that improves virus function. The large gap between SARS-CoV-2 and other viruses to which polyclonal immune responses has occurred in humans further suggests that it is a result of lab processes.
Additionally, (1) the presence of a furin cleavage site missing in other CoVs of the same group and (2) an receptor binding domain (RBD) optimized to bind to human cells[2] might be the result of lab manipulation techniques such as site-directed mutagenesis. The acquisition of both unique features by SARS-CoV-2 more or less simultaneously is less likely to be natural or caused only by cell/animal serial passage. Given this discovery, in an article released August 2020, some researchers created a variant SARS-CoV-2 virus in a lab that removed this furin cleavage site that was unique to SARS-CoV-2. “To explore this question, we generated a SARS-CoV-2 mutant lacking the furin cleavage site (ΔPRRA) in the spike protein. This mutant virus [we created in the lab] replicated with faster kinetics and improved fitness in Vero E6 cells.“. While Fauci would likely deny that this was “Gain of Function”, the semantics are secondary to the fact that this lab created virus – after the pandemic started – that resulted in a variant with “improved fitness”.
A letter from Lawrence Tabak, the National Institutes of Health’s principal deputy director, to Rep. James Comer (R-Ky.) confirms that the NIH funded research at the WIV during 2018-2019 that manipulated a bat coronavirus called WIV1. Researchers at the institute grafted spike proteins from other coronaviruses onto WIV1 to see if the modified virus was capable of binding in a mouse that possessed the ACE2 receptors found in humans — the same receptor to which SARS-CoV-2 binds. The modified virus reproduced more rapidly and made infected humanized mice sicker than the unmodified virus.
Up until recently, the NIH website had a section that discussed gain of function research, providing a broad definition of “a type of research that modifies a biological agent so that it confers new or enhanced activity to that agent.”. On Oct. 20, the NIH removed that section from its website, replacing it with one that discusses “enhanced potential pandemic pathogen” research, which it defined as “research that may be reasonably anticipated to create, transfer or use potential pandemic pathogens resulting from the enhancement of a pathogen’s transmissibility and/or virulence in humans.”
Sadly, experiments continue to synthetically create new viruses that could be dangerous should they escape a lab. A study published October 2021 stated: “The DNA sequences of codon-optimized SARS-CoV-2 Spike RBD (S-RBD) and human ACE2 extracellular domain (hACE2-ECD) were cloned into a pFuse-Fc expression vector (Invivogen). A thrombin cleavage sequence was inserted between the RBD and Fc to generate a cleavable hFc tag for future studies.“.
Tutorial Reading
Here are some “tutorial level” respiratory coronavirus infection and immune response understandings intended as an overview that are not perfect but helpful as a starting point for further learning.
A person exposed to a respiratory viral load has a probability of becoming infected that increases with the amount of the viral load. When a cell becomes infected it reproduces the virus on the order of 1000 to 10000 fold before the cell dies; the first of which are “birthed” after 6 hours or so, resulting in an exponential growth of viral population. After the first generation of virions bud out of the cell, some are “birthed” externally back into the respiratory tract where they can infect other cells by moving through the airway. Some are birthed almost directly into nearby cells. Some are birthed into interstitial fluids that travel via the lymphatic system into the lymph nodes, and usually almost none make it into the blood circulatory system. The virions that contact B/T cells (immune cells which freely travel the circulatory/lymphatic system, but are strongly concentrated in the lymph nodes), activate an adaptive immune response. The sooner this activation occurs, especially activation within the lymph nodes, the more rapidly that adaptive immune response can begin maturing, which produces helpful IgM in about 5 days and much more helpful IgG that increases maturity / specificity over a period of a few weeks. So the race between exponential viral replication vs adaptive immune response maturation and exponential immune response growth can dramatically impact symptoms and pathological damage outcomes. Given exponential viral growth, hours and days can result in dramatically different outcomes. Exercise and anything else that speeds the adaptive immune activation and maturation is usually very helpful to improving the immune response before the exponential viral growth becomes larger and more widespread. Prior immune response (caused by prior infection or vaccination) dramatically improves response time because B/T cell immune response maturation and antibody secretion has already occurred and can be re-activated within several hours depending upon exercise levels, etc.
A larger initial exposure viral load can cause increased severity because the exponential viral replication reaches much higher levels before the immune system can respond. This results in dramatically greater viral damage. The increased viral presence also tends to activate a more intense immune response which may result in increased systemic damage from the immune response. Reducing viral loads into a variolative or minor infection range can significantly reduce the likelihood of a severe case. A regular regimen of a few cycles of normal daily exercise improves immune response / speed pre-exposure and immediately following exposure to minimize likelihood of illness.
It is possible to minimize severity of illness by slowing the exponential growth of viral load that develops shortly after exposure which helps the adaptive immune response to mature before exponential viral growth becomes extremely large. The innate immune response tends to slow natural infections; and intelligent behavior (such as using viral inactivation substances such as mouthwash, toothpaste, essential oils / breath-mints / breath-strips, zinc, small amounts of ethyl alcohol, etc.), can slow the exponential growth such that B/T cell activation /maturation occurs before exponential growth of antigen becomes large. Mints and essential oils have been recognized as helpful for many centuries, and many modern mouthwashes that kill bacteria and inactivate viruses are now essential oil rather than alcohol based. Swishing fractions of an ounce of high-percentage ethyl alcohol (hard liquor) into the throat area with tickle/minor-irritation every few hours tends to slow viral growth, although once viral damage to the tissues becomes significant, this tends to create further irritation / tissue damage; and larger amounts of ethyl alcohol may negatively impact immune system response, so this tends to be optimal only for very early “tickle” stages of illness. Placing 1/16th of a zinc supplement (nibble off a small piece) and/or a few grains of salt in room temperature or slightly warm water will make the water much more pleasant to drink, which may help you to remain well hydrated. The zinc also seems to have a throat soothing effect (try it) and may have some mild topical protective characteristics and this tends to be effective in both early and later stages of illness. Just a nibble of a zinc mineral supplement and a few grains of salt go a long way and also tend to make water less irritating to the sore throat – thus encouraging increased hydration which is also helpful. Salt water gargling tends to be helpful. Swishing saliva around within the mouth and onto irritated areas helps to keep them moist and in some cases will help mucosal antibodies find and bind to viruses that may be present near the irritated area that is replicating virus. This can be particularly helpful during re-exposures and in later stages of infections where significant virus specific antibodies may be present within the saliva. Histamine responses also tend to increase likelihood of antibody encounters with viruses within mucosal tissues and also help to purge virus from the body through sneeze and runny nose responses.
It is likely that many infections and transmissions can be avoided or minimized through the use of breath-mints / breath strips during and following potential exposure encounters.
When sick, highly aerobic activity can undesirably facilitate virus movement deeper within the airway and into the lungs, yet light exercise such as walking can stimulate blood/lymph flow without resulting the deep respiration that internally spreads virus within the airway. Similarly, fresh air and avoiding rebreathing “sick-room” viral laden air can help avoid spreading the infection within the airway. Perhaps obviously, keeping considerable physical distance from others while enjoying fresh outdoor air will dilute any viral load that might be shedding when sick and wearing masks can help while in careful transit to some wide open personal space containing fresh air.
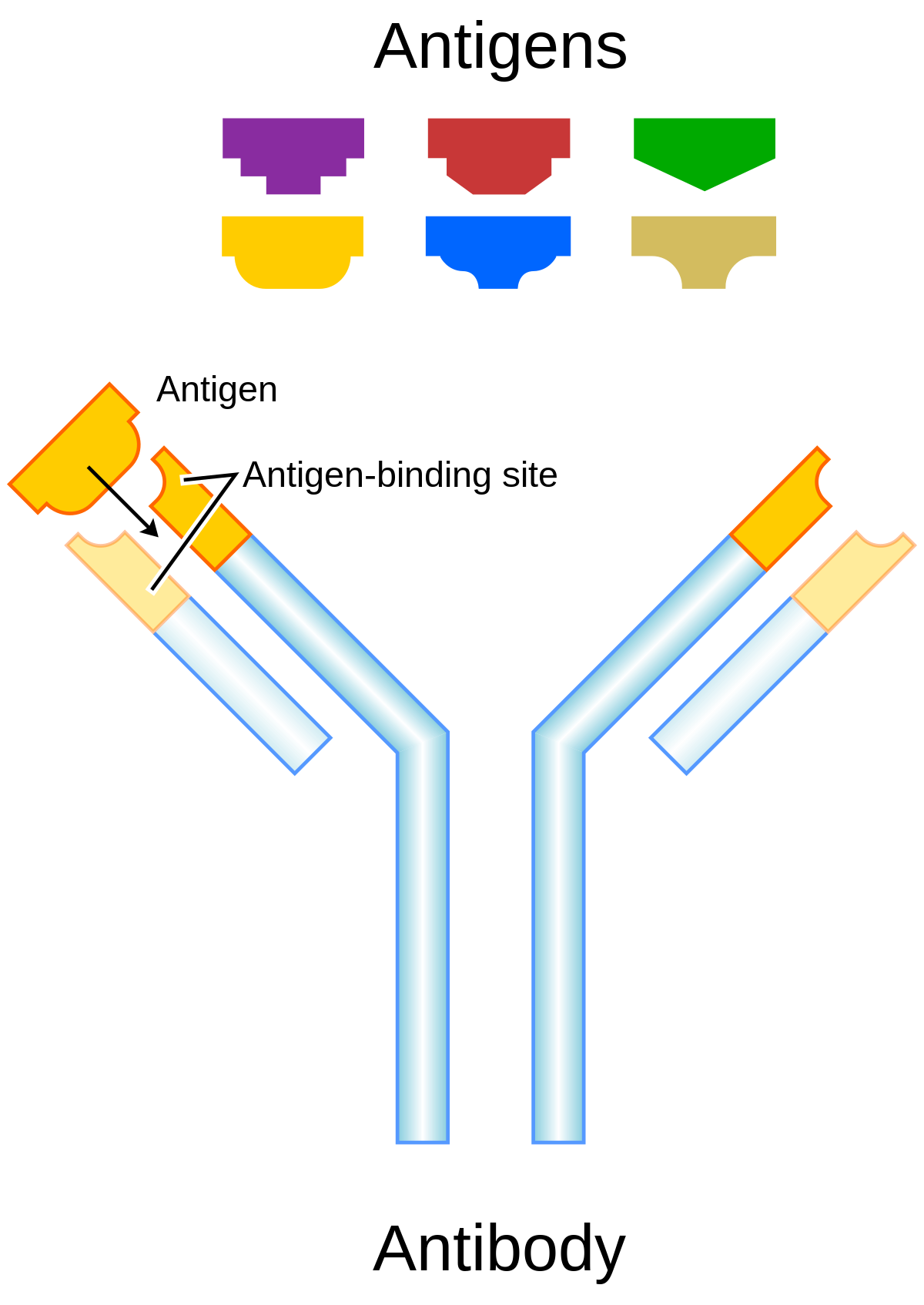
Antibodies “neutralize” and/or “tag” coronaviruses by “binding” to the virus “spike protein” and thus occupy the spike area that tries to attach to a cell and invade the cell. Antibodies are created by B cells that have matured in to B “plasma” cells that are specific to the virus. B cells are “naive” and are not specific to a particular virus until they encounter the virus and begin a “maturation” process. Naive B cells do weakly recognize many viruses (and bacteria) but do not become strongly “binding” / “sticky” until they complete this “maturation” process. Once B “plasma” is mature, it secretes virus specific antibodies that strongly bind to the specific virus and neutralize it and/or tag it so that phagocytes may more easily find and “eat” it.
Both infection from the coronavirus and vaccination will, in normal physiology, result in the development of virus specific B cells which include B memory cells and “B” plasma cells which develop when a “naive” B cell becomes activated (essentially though a random encounter with the virus – usually within the lymph node), undergoes a “maturing” class shift, becomes “plasma” and produces virus specific antibodies. Similarly naive T cells become activated and become specific to the virus. Together, the plasma, and antibodies produced by the plasma, and the T cells offer a defense against the virus. Over time, the plasma cells “die” (apoptosis), and thus stop secreting antibodies. Antibodies also are eliminated over time / breakdown usually before 20 days or so, but remaining B plasma and LLPC’s continue to secrete “fresh” antibodies. There’s some “magic” not fully understood that causes Bmem and Tmem to avoid apoptosis and/or result in some “homeostatic maintenance” function that keeps them around for a very long time – lifelong level in many cases.
When Bmem that is specific the the virus re-encounters the virus (potentially much later – re-exposure), they divide into more Bmem and plasma and the new plasma secretes antibodies. This is kinda neat in that we aren’t clogged with antibodies to everything we’ve encountered forever, but can generate them again when re-exposed. And perhaps it should be mentioned that the plasma has class-shifted into various types that create different antibody isotypes including IgA and IgG. A semi-mature plasma cell version that usually exists significantly during the initial primary infection presents/secretes IgM. That’s the first thing that a naive B cell exposed to a virus produces as it begins to mature and become specific, but it eventually continues it’s class shift into an IgA, IgG or one of the other isotype secreting plasma cells. There are more nuances to this – please research it further in the links below. With each re-exposure, and generally over the course of primary infection, encounters with the virus tends to lengthen and strengthen immunity. It does this by increasing the specificity / binding-affinity / stickyness of the antibody paratope (that mates to a matching virus epitope) as well as increasing the population of virus specific B plasma, B mem, T helper, T killer/cytotoxic and T mem cells and possibly some improvement in the “magic” that keeps them around for the long term (research Long Lived Plasma Cells (LLPC), bone marrow, and homeostatic maintenance). So, a vaccine, given 2x is effectively a re-exposure. Similarly, in an outbreak, someone who has recovered would likely re-encounter the virus at a later date. Interestingly, this mechanism seems to strengthen immunity during each encounter. So something that stays around or comes around often would be more likely to result in long-term immunity – very amazing system!
From Wikipedia Affinity maturation: “In immunology, affinity maturation is the process by which TFH cell-activated B cells produce antibodies with increased affinity for antigen during the course of an immune response. With repeated exposures to the same antigen, a host will produce antibodies of successively greater affinities. A secondary response can elicit antibodies with several fold greater affinity than in a primary response. Affinity maturation primarily occurs on surface immunoglobulin of germinal center B cells and as a direct result of somatic hypermutation (SHM) and selection by TFH cells.[1]“
Summarizing the cellular immune components, T cells have different types such as CD4+ T helper which assist in the activation of B cells as mentioned, and CD8+ T killer which detect virally infected cells within the body and kill them so they don’t keep replicating more viruses.. Antibodies like IgM, IgG, and IgA neutralize a virus by preventing it from entering a cell and also tag the virus so that phagocytes, etc. will identify the antibody-tagged virus and “eat” it. More can be learned by researching the adaptive, innate, and complement immune system components in the articles below.
Protection From Vaccines And Natural Infection
Some articles assert that vaccines provide better long-term immunity. Most vaccines (certainly mRNA) do not result in any viral infection and only activate the B/T cells. Activation is the first step of a process that converts B/T cells from naive to specific. Vaccines activate but do not actually result in an infection, so some make a case that the vaccine can contain a greater “load” of antigen epitopes (but not the rest of the virus) which makes a larger B/T population; however, a vaccine dose that is not reasonably matched to an individual’s physiology can result in excessive immune response / adverse events. A significant exposure to the virus typically results in B/T/antibody populations similar to that of vaccine; however, viral exposure is often accompanied by viral damage, sometimes severe, and sometimes a severe immune response that can be very harmful. Vaccines commonly may have mild systemic side-effects (in addition to injection site pain) which are mostly related to the immune response – not the vaccine components, and may sometimes create significant adverse events, and extremely rarely cause ADE which is usually caught during vaccine trials and the vaccine is then not approved for use. In general a properly dosed, timed, physiology-matched, properly injected, properly manufactured and stored vaccine is often a lower risk approach to developing protective immunity than natural infection, but vaccines are not free of risk as many variables accompany injected substances. An individual having recovered normally from COVID prior to vaccine availability is likely to have substantial protective immunity that is comparable to vaccination. Re-exposure can be important to length and strength as mentioned earlier. In epidemics, that re-exposure is likely to occur within the context of normal daily activities, or could be provided by a vaccine booster many months later.
So which provides better long-term protection, vaccine or recovery from natural infection? While mRNA and other vaccines may create a very diverse polyclonal antibody response, encountering the virus often results in more diverse immune response because the mRNA usually does not create proteins for all aspects of the virus to include all of the S/RBD, M, E, and N proteins. Most mRNA vaccines are designed to create a currently-thought best set of proteins to stimulate immune response. For example, the Moderna and Pfizer vaccines approved in December 2020 encode the entire spike that includes the highly important S/RBD proteins. These mRNA vaccines do not encode the Membrane, Envelope, or Nucleocapsid proteins and thus antibodies to those proteins are not developed. In fact, testing for E and N antibodies can reveal whether a natural infection has occurred vs the S antibodies that accompany both natural infection and vaccine responses. With antigen level and all other things being equal, the RBD neutralizing effectiveness would likely be equal between natural infection and vaccine response. However, all other things being equal, the natural infection response would tend to be more protective because the more diverse immune response with M, E, and N antibodies would be more likely to “tag” the virus for phagocytosis and other complement immune response in addition to the RBD neutralizing antibodies shared by both natural infection and vaccination.
If the antigen level profile over time was held “theoretically” identical between vaccine and natural infection, natural infection would have a more diverse and thus more protective result. For natural infections where more antigen developed during exponential replication before an adaptive immune response scales, relative to the vaccine case, it is likely that a stronger immune response and better protection would develop as a result of natural infection. In the case of a natural infection exposure with lower antigen levels than that provided by vaccine, the greater natural infection immune response diversity would be offset by a lower overall level of antigen providing activation of adaptive immune response, and would likely result in lower protection than the vaccine response.
Said another way, it is likely that asymptomatic or lightly symptomatic natural infections that have symptoms more mild than the typical 1 day dose 2 side effects of myalgia, fatigue, headache, chills/fever, etc., will result in lower protection than the vaccine. Natural infections with greater symptoms than the dose 2 side-effects are likely to have stronger protection than the vaccine. And, with all of this, there is also some bias in favor of natural infection due to the more diverse immune response. This will not always be the individual case, but over a broad population, this correlation would likely exist.
Further, there is some evidence that the N antibodies that result from natural infection, but not S/RBD targeting vaccines, help reduce severe outcomes from SARS-CoV-2 infection / exposure. A study stated “Our work indicates that human N-targeting mAbs from COVID-19 convalescents play essential roles in inhibition of complement hyperactivation.” and “these data suggest that hyperactive complement predispose individuals to adverse outcomes associated with SARS-CoV-2 infection.”
Some articles claim immune response from vaccines is better than natural infection response. Again, it likely depends on infection severity and other parameters vs the “endgame” of vaccination adjuvant behavior that can leave a temporary spike in antibodies but not necessarily an overall stronger / longer immune response, but possibly so. There are many nuances and complexities. Epidemiological data appears to show Israelis who were vaccinated were 6.72 times more likely to get infected after the shot than after natural infection. Data is mixed. People who have recovered from natural infection need to weigh benefits vs risk of a vaccine booster based on their specific physiology and risk tolerance. An Israeli study released in August 2021 stated SARS-CoV-2 naïve (people who never had COVID) vaccinees had a 5.96-fold increased risk for breakthrough infection and a 7.13-fold increased risk for symptomatic disease. SARS-CoV-2-naïve vaccinees were also at a greater risk for COVID-19-related-hospitalizations compared to those that were previously infected, suggesting that natural infection, across a population, affords better protection than vaccine. The finding in the Israeli epidemiological study is consistent with what would be expected given immunological understandings.. Given the typical symptoms that follow a personally observed and/or clinically diagnosed mild infection, most asymptomatic infections, which may result in less protection than vaccine, are typically not observed / diagnosed and therefore the individual is unlikely to make a claim of natural infection, which further strengthens the case that observed / diagnosed natural infections would most often lead to better protection than the vaccine.
A study of 52238 employees in the Cleveland Clinic Health System stated that “COVID-19 did not occur in anyone over the five months of the study among 2579 individuals previously infected with COVID-19, including 1359 who did not take the vaccine.” and concluded that “Individuals who have had SARS-CoV-2 infection are unlikely to benefit from COVID-19 vaccination, and vaccines can be safely prioritized to those who have not been infected before.“
Another study showed SARS-CoV-2 infection induces greater T-cell responses compared to vaccination in solid organ transplant recipients.
Interestingly, vaccine antigen level vs time profiles are considerably different than natural infection. A vaccination provides a relatively fixed amount of antigen that then disperses into circulation over time and diminishes; all of this being modified by adjuvant that is within the vaccine dose. Natural infection antigen level vs time profiles tend to start very small, typically well below activation levels, and grow exponentially until the adaptive immune response becomes activated. With normal amounts of physical activity, which helps the antigen reach lymph nodes where activation of adaptive immune response is most rapid, the adaptive immune response activates, halts, and reverses the exponential growth. The interplay of these different antigen level vs time profiles for vaccine and natural infection are of great intrigue as the vaccine tends to start large and decrease over time, while natural infection starts well below activation thresholds and often rapidly grows to activation threshold levels. Innate immune response tends to slow natural infections; and intelligent behavior (such as using viral inactivation substances such as mouthwash, toothpaste, essential oils / breath-mints, zinc, small amounts of ethyl alcohol, etc.), can slow the exponential growth such that activation occurs before exponential growth of antigen becomes large. Mints and essential oils have been recognized as helpful for many centuries, and many modern mouthwashes are now essential oil rather than alcohol based. It could be argued that for many non-life-threatening viruses, intelligent behavior and natural immune response is superior to a relatively fixed vaccine dose immune response. The fixed amount vaccine dose may be insufficient to establish a strong immune response in some individuals or may be too large and create an adverse event in other individuals. Natural infection and the viral replication growth tends to result in “finding” the individual’s immune response activation threshold such that the immune response results in stronger future protection; although that experience is generally unpleasant, or worse. Vaccine priming at low doses can be quite helpful for mitigating the unpleasant effects of a future infection, unless the vaccine dose is so large for the individual that it results in an adverse event. Research continues on adjuvant behavior in an attempt to optimize vaccine antigen level vs time profiles, etc.
The Risk Of Over-Boosting / Vaccination
Keeping in mind homeostatic processes at work within the body that maintain appropriate levels of immature naive B/T cells, mature, virus-specific, B memory cells, T cells, LLPC’s and the entire immune complement, it is possible to create imbalance through excessive boosting of immune response to a specific virus. Creating a narrower set of B / T / antibody “species” that strongly bind to one specific virus can “push out” a more diverse set of polyclones that provide broader protection against a wider set of viruses. Conceivably, it could mute vaccine induced immune responses to other, potentially more pathologic viruses, and reduce protection against those pathogens, such as measles. And for a period of time near the boosting, it could reduce availability of naive B cells which are important for developing immune responses to previously unencountered viral / bacterial exposures. Over-boosting could also result in allergic / adverse events through over-sensitization that could span a set of pathogens / substances beyond that of the target virus. It could result in aberrant behavior in the delicate B/T cell activation process and enable development of larger auto-antibody populations and associated auto-immune disease. Certainly the notion of 3 and 4 vaccinations within a 1 year time period for general population distribution is not well tested. It may not even be prudent for the immuno-compromised / geriatric at strong dosages. The covid-era passion for prime vaccination and boosting appears to be driven by factors that do not include a thorough consideration of benefit vs. risk across a broad perspective and timespan.
Bound Antibodies And Immunoassay Uncertainty
Regarding measurement of antibodies, it is important to note that immunoassay techniques can result in erroneous measurements. Immunoassays that measure antibodies by binding a “tag” or “label” to the antibodies (and then looking for a fluorescent tag’s light emission), show lower counts when the antibodies are bound to virus (which is how antibodies neutralize the virus). Early in an infection, there tends to be much more virus present than antibodies, as the antibody secretion by B cells ramps up. Statistically speaking, this means that many of the antibodies are bound to virus when the population of virus is much larger than the population of antibodies. This results in the immunoassay showing that there are many fewer antibodies present than actual when that virus / antibody population mismatch is large. Only when they become more even are there enough free “unbound” antibodies such that they are measured. Substances that alter the measurable concentration of the analyte or alter antibody binding can potentially result in immunoassay interference. The immunoassay is measuring the amount of “unbound” antibodies, so this is important to keep in mind as it creates the appearance that antibodies are developing later in time than they really do. Early on in an infection/vaccination, many of the antibodies are “bound” and are thus not detected because the immunoassay tag cannot bind to them as the binding area is already occupied by virus. This also helps explain why antibodies “appear” to have peak populations some time after an infection “clears”, because at that time the reverse condition occurs: there are many antibodies, but very little virus that are bound to the antibodies, since the virus has been eradicated from the body. The reality is that many antibodies exist and probably genuinely peak when there is a closer match of virus / antibody populations (actually several antibodies can bind to the multiple spikes / epitopes on a single virus, so it’s more of when the number of total viral spikes and antibodies match). There are many complexities, but this helps one understand some important concepts of antibody measurement and errors that occur during that measurement. It also helps one understand the “war” as antibodies bind to the virus and neutralize the virus by preventing the spike from attaching to a cell simply by occupying the spike that is trying to attach to the cell.
Regarding vaccine dose timing, the 2nd dose antigens have a probability of binding to any antibody titer remaining from the dose 1 immune response, preventing the antigen from interacting with B / T cells which is necessary to improve binding affinity (immunity). An appropriate delay allows antibody response to wane from it’s peak and enables a higher probability of B / T / antigen re-activation and further SHM responses within germinal centers. Existing B / T mem, antibodies, etc. remain protective during the period between doses.
Sterilizing Immunity
It can also be shown from general immunological principles that a level of sterilizing immunity generally arises when IgA, CD8+ T cytotoxic “killer” cells, and IgG are present (see: ref1, ref2, ref3, ref4, and above/below). Regarding re-infections, if IgA/IgG antibody titer and T killer cell population has diminished after a primary encounter many months / years ago, a re-exposure that is successful at entering some cells will technically result in a re-infection. But the adaptive immune arsenal that is remaining will provide “protective immunity” and rapidly halt the re-infection which will often be short lived and most likely asymptomatic or result in minor symptoms; and shedding is often minimal to none; all of this depending on the armies on each side, so to speak. Since virus specific B / T mem already exist during a reinfection, the process of rebuilding a new population of plasma cells and antibodies is very fast (hours to a day or so, somewhat depending upon exercise level, etc). If a primary immune response has occurred within the last several months (from vaccination or natural infection recovery), it is likely that plasma, IgA, IgG, and Tc will be present in quantities that provide instantaneous protection. Since IgA is typically found in the mucosal areas, saliva, gut, etc., having lots of IgA, and T killers, can result in a level of “sterilizing immunity”, such that any viral load has no ability to create an infection substantial enough to result in any significant viral replication / shedding. Being continually re-exposed can keep IgA and T killers nicely populated so that the individual kills the virus rapidly and provides little threat to others (how smart is that?). In fact, they potentially become better than a “surface” as viruses can remain viable on some surfaces like stainless steel, glass, and plastic for quite a long time but the IgA would neutralize them by binding to the epitope and blocking / preventing operation of the spike protein in it’s attempt to bind to a cell, so even if they somehow sneezed or breathed the encountered virus back out, if it was in the nose long enough to encounter IgA and become neutralized, it would leave neutralized. An individual with strong IgA / mucousal antibody presence not only doesn’t replicate virus, but can act as an “air filter” that removes and/or inactivates virus that is present in the environment, similarly to a dog instinctively (or perhaps knowingly) licking wounds. Also see Biochemical Composition of Human Saliva in Relation To Other Mucosal Fluids (1995). Is it possible that a dog’s instinct is keener than that of some COVID-19 policy makers?
Polyclonal Immune Response
A virus has many different epitopes on it’s spike. During immune response, many different antibodies develop to match-with and bind-to these different viral epitopes on the spike. Thus, the immune response creates polyclonal antibodies that work together to neutralize the virus. The more viral epitopes that become bound with matching antibody paratopes, the better neutralized and tagged the virus becomes. Some virus epitopes, for example the ones on the virus’s spike Receptor Binding Domain (RBD) tend to be very important to infecting a cell, and “neutralizing” antibodies that bind to those epitopes tend to be most effective at preventing infection by halting the cell from absorbing the virus, essentially by “capping” that area of the virus. The majority of the epitopes on the virus are not part of the cell infection mechanism, but non-neutralizing antibodies which bind to these epitopes will “tag” the virus and make it more attractive to phagocytes which will then “eat” the virus. The team of diverse polyclonal antibodies all work together to reduce the chance of the virus being able to infect a cell as they “tackle” the virus from every “angle” (neutralize and/or tag it via binding to multiple epitopes). Perhaps obviously, when more antibody polyclones become bound to the various virus epitopes, the greater the likelihood that it will be neutralized, unsuccessful at invading a cell, and become “eaten” by phagocytes, etc. (phagocytosis).
A variant virus will have some epitopes that are identical to the earlier virus that formed the earlier immune response and some epitopes that are different. The epitopes that are identical (or nearly so) can bind to existing antibodies; and they can also activate existing B mem and this usually happens within hours. Epitopes that are different will cause new B mem and antibodies to develop in a period of several days. In the case where the pre-existing clonal types rapidly halt virus progression, it is less likely that new clonal types will develop – a concept known as “original antigenic sin“. When the variant becomes different enough (or a larger initial exposure) such that it cannot be rapidly halted with the original clonal types, new clonal types will develop alongside a likely more symptomatic illness.
As a virus mutates, the strength of antibody binding, and number of antibody monoclones/”polyclonal types” that bind, can diminish, usually a little more with each mutation of the various viral epitopes. “Nearly the entire surface of an antigen presents many overlapping domains that antibodies can discriminate as distinct epitopes”. After many mutations (usually years later), several of the various polyclonal antibodies may be of little help; that said, several of the polyclonal antibodies will remain effective at neutralization.
During encounter of a variant virus, the epitopes in common with the original virus will be rapidly bound by existing antibodies and more of those same antibodies will be rapidly secreted by B plasma cells that rapidly proliferate when B mem cells are activated within hours of the re-encounter. This will either rapidly halt the reinfection or significantly slow it depending upon how much and how many of the variant virus epitopes mutated, etc. Even slowing the new infection helps to thwart viral damage while the new immune response adapts to the variant. Slowing the variant caused infection can dramatically reduce the otherwise rapid exponential viral growth that occurs in a person that is completely naive to the virus.
Encountering variants / mutations over the years causes development of more specific B mem and antibodies that bind to the viral epitopes that have mutated. So, the older B mem encodes antibody paratopes that bind to the “old” epitopes and new B mem becomes specific to the newer mutated epitopes. A gap where mutations are missed (no exposure) or a large sudden mutation can make older B mem less protective. Encountering mutations along the way will likely result in asymptomatic infection or minor illness while refining the binding affinity to the newer mutations, creating future protection. Thus, serious infections from a recent variant are quite rare.
“Each B cell produces a single species of antibody, each with a unique antigen–binding site. When a naïve or memory B cell is activated by antigen (with the aid of a helper T cell), it proliferates and differentiates into an antibody-secreting effector cell. Such cells make and secrete large amounts of soluble (rather than membrane-bound) antibody, which has the same unique antigen-binding site as the cell-surface antibody that served earlier as the antigen receptor (Figure 24-17). Effector B cells can begin secreting antibody while they are still small lymphocytes, but the end stage of their maturation pathway is a large plasma cell (see Figure 24-7B), which continuously secretes antibodies at the astonishing rate of about 2000 molecules per second. Plasma cells seem to have committed so much of their protein-synthesizing machinery to making antibody that they are incapable of further growth and division. Although many die after several days, some survive in the bone marrow for months or years and continue to secrete antibodies into the blood.”
How many different clone types are created in response to the virus or vaccines based on the spike? “A single antigen can be thought of as a sequence of multiple overlapping epitopes. Many unique B cell clones may be able to bind to the individual epitopes. This imparts even greater multiplicity to the overall response”. One study, looking only at the just the RBD portion of the spike found “the isolation and characterization of 206 RBD-specific monoclonal antibodies derived from single B cells from 8 individuals infected with SARS-CoV-2“. So there are probably many more they didn’t isolate and a large number of additional monoclones that bind to the numerous epitopes along the entire spike. Also see “Mining the Antibody Repertoire for Solutions to SARS-CoV-2“. Additionally, there is “evidence from AZ trial that CD4/CD8 cells respond to 87 epitopes“. Another study isolated 1737 monoclonal antibodies (mAbs) from a SARS-CoV convalescent patient 17 years following infection and from a SARS-CoV-2 convalescent patient 36 days post infection and found 50 cross-reactive antibodies that bound to SARS-CoV, SARS-CoV-2, and other human and animal coronavirus antigens. Natural infection often results in over 1000 unique antibody / virus-specific-B-cell “species”. Every antigen, H1N1, SARS-CoV-2, etc., elicits polyclonal immune response. Cross-reactivity results when an individual B/antibody species binds to multiple viruses & either tags or neutralizes them. Immunity to hundreds of viruses improves cross-immunity. While using NPI’s such as masks, physical distance, etc. reduces potential for infection, this also increases the gap in adaptive immune response capability due to the lower likelihood of polyclonal cross-immunity. How isolation measures may impact a population decades later is not well understood; however, the implications of immune response gaps are somewhat obvious.
“Transition of B cells to plasma cells is associated with loss of BCR expression. In the absence of opposing BCR signaling, FcγRIIb engagement by IgG immune complexes leads to plasma cell apoptosis; this negative feedback mechanism regulates plasma cell survival to prevent uncontrolled IgG production.“
For infectious diseases where immunization can offer lifelong protection, a variety of simple models can be used to explain the utility of vaccination as a control method. However, for many diseases, immunity wanes over time [years and decades] and is subsequently enhanced (boosted) by asymptomatic encounters with the infection.
Increasing the population of vaccinated / natural-infection-recovered individuals will reduce the potential for variants to occur by reducing the total number of viral replications. Vaccine resistance is rare due to the diverse polyclonal immune response. That said, it is likely that variants with significant mutations will produce asymptomatic or minor infection as indicated above. One study showed SARS-CoV-2 specific memory B-cells from individuals with diverse disease severities recognize SARS-CoV-2 variants of concern.
The course of the SARS-COV-2 pandemic in the UK seems highly predicted by an earlier model based on the lockdown stringency, humidity and temperature and unaltered by the emergence of a newer viral genotype. Vincent Racaniello, a professor of microbiology and immunology at the College of Physicians and Surgeons of Columbia University stated: there’s no evidence so far of a variant that can meaningfully evade vaccines, though there are several variants out there. Monica Gandhi, MD MPH – infectious diseases and HIV doctor at UCSF, states: “Let’s remember from papers tweeted before, immunity from natural infection & vaccinations across viruses lasts long”.
Most variants such as B.1.117 and P1 that are frequently touted as being more transmissive became prevalent during periods of opening and/or holidays, and it is quite possible that observed increases of spread were due to human behavior such as increased indoor activity which likely leads to greater R0 and increased initial exposure viral load encountered indoors. The one variant, B.1.351, that seems to have some significant level of in-vitro escape in some cases, appears unlikely to strongly escape in-vivo adaptive immune protection for the reasons mentioned above. Blood / plasma samples mixed with the virus outside the body understandably have much weaker neutralizing activity than virus in a live body with lymph nodes and a fully responding immune system, so the studies that are based on neutralization assays are not necessarily reflective of actual in-vivo human responses that are quite robust as discussed above. Real world data regarding B.1.351 escaping immune response has not been convincing to date.
Co-circulating Variants And Sequencing Bias
As variants develop, it becomes more likely that a diverse set of variants could co-infect a population. It becomes interesting to better understand how PCR amplification and genetic sequencing methods could introduce biases into the determination of the variants identified by sequencing when an individual is co-infected and a sample is submitted for sequencing. New genome sequencing technologies [1–3, 15–17] no longer rely on cloning in a microbial host. Instead of ligating DNA fragments to cloning vectors, the three major platforms currently on the market (454, Illumina and SOLiD) involve ligation of DNA fragments to special adapters for clonal amplification in vitro rather than in vivo. Due to the massively parallel nature of the process, standardized reaction conditions must be applied to amplify and sequence complex libraries of fragments that comprise a wide spectrum of sequence compositions. All three platforms display systematic biases and unevenness as the observed coverage distributions are significantly wider than the Poisson distribution expected from unbiased, random sampling. Having a better understanding of how amplification and sequencing methods create variant identification bias when a population genuinely has multiple circulating variants is of some interest. For example unlike targeted qRT-PCR assays, virome capture sequencing can be used to determine the genome sequences of coinfecting viruses, informing investigations of virus transmission and evolution. … Although the amplicon approach can also construct libraries within a similar timeframe, in our experience of using two published amplicon WGS methods, generating amplicons often took longer than anticipated due to certain parts of the genome amplifying poorly, requiring continuous optimization. Understanding and correcting for any bias associated with specific co-infection variants would be important to assuring that sequencing results most closely match the underlying reality as all measurement processes are subject to uncertainties. A study provided laboratory data showing how mutations disrupted sequencing of P.1 lineage specimens during a recent outbreak in British Columbia, Canada, and we also demonstrate how we modified the Freed et al. protocol to restore performance.
Popular reporting about the B.1.617.2 (Delta) variant commonly states this variant is replicating more rapidly and displacing other variants, but since it is supposedly displacing other variants, this is extremely likely to be occurring in an environment that has multiple variants co-circulating and likely that many co-infections develop as a result. It is not clear how current genetic sequencing of infection samples resolves co-infections from multiple variants to support the popular press claims. In fact, one March 2021 Cell Press article states that “to date, no method can rapidly diagnose multiple viral infections and determine variants in a high-throughput manner“. While there are many assertions that selection of variants is occurring biologically within a population where multiple variants are co-circulating, the question arises regarding whether that selection is genuinely from the population, or whether the “appearance of selection” is an artefact of an amplification and sequencing process bias when applied to a sample from a co-infected individual. In other words, if a person is co-infected by 2 or more variants, what’s the sequencing result? Are all variants in the sample reported? Just some? Only one? Which “wins”? Does sequencing bias errantly determine the “winner” rather than accurately reporting proportion of variants in the sample?
In review, antibodies are secreted by B “plasma” cells. Most immunologists just call them plasma cells or PC. It’s helpful to trace it back to it’s roots as naive B cells which are created in the bone marrow regions. In fact, T cells are also built from premature versions of B cells and they mature in the Thymus. T cells don’t secrete antibodies. They help activate B cells and also form T killers mentioned above. And, there are on the order of 10,000 (or maybe a few orders of magnitude more) “random” types of naive B cells, each being a little more or less likely to bind with different types of viruses and bacteria. Of those “10,000”, only a few are likely to recognize and loosely bind with any given virus. Those few are the ones that mature into B plasma when they encounter the virus and become activated as mentioned above, and so on.
The reality can be significantly different than stated here depending on the precision desired. Additional resources below and elsewhere can help clarify, correct, and extend this tutorial information.


“This rapid and dramatic antibody response may stop the infection before it can even become established, and the individual may not realize they had been exposed.”
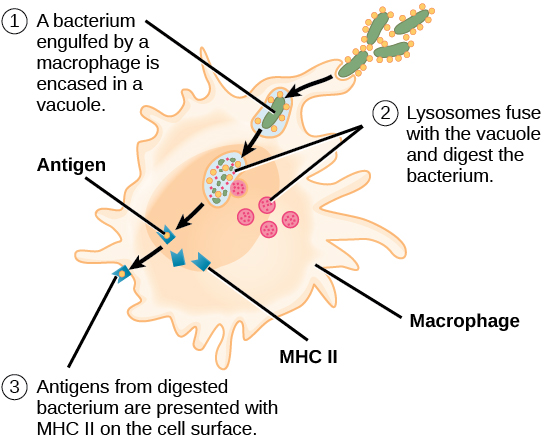
“Repeat exposure has the effect of refining IgG affinity of the antibodies and boosting the number of memory cells.” -MD, EpGrp
“Antibodies are made by B cells; T cells can be helpers (Th) or cytotoxic (Tc) but don’t make antibodies. Each B cell has a unique receptor for a single epitope. When B cells encounter antigen the ones with receptors that bind with a certain affinity begin to proliferate and mature, first secreting IgM then undergoing a class shift and maturation to plasma cells that secrete IgG and IgA depending on the cytokine milieu and other factors. Since this is a population effect the final antibody titre will depend on the numbers and activity of the plasma cells. During maturation some cells become Bmem but the antibody titre probably doesn’t depend on those; they are held in reserve in case of repeat exposure to the antigen.” -SW, EpGrp
“This essentially answers the question that Bmem and IgG antibody titer are not necessarily correlated. And further, that the Bmem is of potentially greater importance than IgG regarding re-challenge. In fact, having IgG just makes the response to the challenge much more rapid as there is no lag while Bmem multiplies and secretes more IgM / IgG in response to the re-challenge. But even that latter Bmem response is still quite fast, just not as fast as having lots of IgG immediately circulating.” -DV, EpGrp
“Homeostatic maintenance of Bmem is sufficient to result in long-term population of antigen specific Bmem / plasma.” -DV, EpGrp
“I know hepatitis b vaccine, you can have immunity even after 30 yrs from vaccine even if low titers or non measurable titers” -FM, EpGrp
“How would our immune system look if we were churning out massive amounts of antibodies to everything we ever encountered? We’d be a giant lymph node.” – DK, EpGrp
Courtesy Emily Watters, M.D. @cartoonshrink



natural and vaccine-induced immunity against this coronavirus and the disease, COVID-19,
that it causes. In this Primer, we explain the fundamental features of T cell memory
and their potential relevance for …
Although detailed mechanics of the immune response are beyond the scope of this site, it is useful, in the context of developing a custom antibody, to have an overview of how antibodies are produced by the immune system.


“After puberty, the thymus starts to involute with age and turns into fatty tissue“




Antibodies typically bind to the virus’ nucleocapsid (N), the spike (S), or receptor binding domain (S-RBD) portion of the spike. Each of these proteins have differing epitopes such that the antibodies for each are different. An antibody which binds to the S-RBD will neutralize the virus by preventing it’s attachment to a cell. All antibodies will attract phagocytes, etc. via the Fc region of the antibody.
“A coronavirus contains four structural proteins, including spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins.2,10,11 Among them, S protein plays the most important roles in viral attachment, fusion and entry, and it serves as a target for development of antibodies, entry inhibitors and vaccines.1,12,13,14,15,16,17 The S protein mediates viral entry into host cells by first binding to a host receptor through the receptor-binding domain (RBD) in the S1 subunit and then fusing the viral and host membranes through the S2 subunit.16,18,19 SARS-CoV and MERS-CoV RBDs recognize different receptors. SARS-CoV recognizes angiotensin-converting enzyme 2 (ACE2) as its receptor, whereas MERS-CoV recognizes dipeptidyl peptidase 4 (DPP4) as its receptor.20,21 Similar to SARS-CoV, SARS-CoV-2 also recognizes ACE2 as its host receptor binding to viral S protein.22 Therefore, it is critical to define the RBD in SARS-CoV-2 S protein as the most likely target for the development of virus attachment inhibitors, neutralizing antibodies, and vaccines.“
“A long-standing debate about whether specific memory is maintained by distinct populations of long-lived memory cells that can persist without residual antigen, or by lymphocytes that are under perpetual stimulation by residual antigen, appears to have been settled in favor of the former hypothesis.”

maturation of B lymphocytes into antibody-secreting cells. In this review, Mesin et al.
update the current understanding of the cellular interactions and positioning of B
lymphocytes within these foci to support a hum…
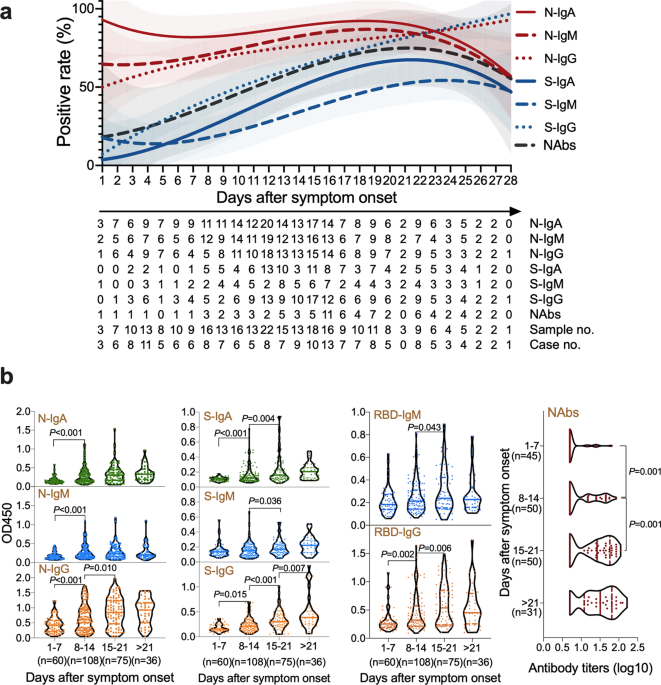






There are some misleading statements regarding 1) reinfection and 2) vaccines always being better than an actual infection; in the overview information, but there are also important details within “Why a vaccine can provide better immunity than an actual infection.” As with all scientific publications, read with care and a critical mind.
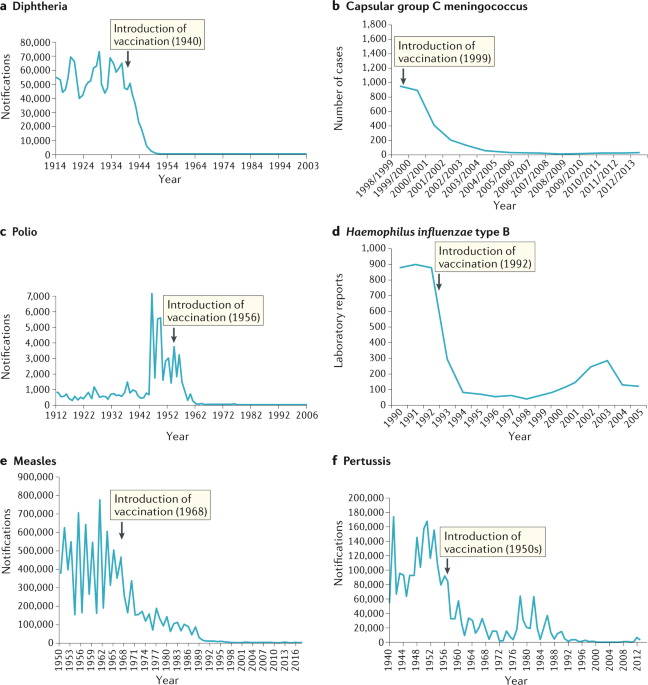



“Memory B cells circulate throughout the body in a quiescent state until specific antigen is re-encountered and triggers a potent secondary immune response. Memory cells respond to antigen much faster, require lower amounts of antigen, and can even be induced in its absence by soluble mediators such as IL-2 or IL-15, in part because the BCR is already localized to lipid rafts. Subsequently, just like naïve B cells, memory B cells ingest antigen and express peptide–MHC class II fragments. After antigen presentation of peptide to helper T cells, memory B cells undergo expansion and may differentiate to plasma cells.”

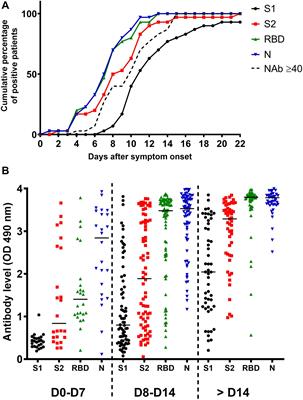
Possible Long Term Immunity From Mother’s Milk
While it is well established that short-term protection of an infant can arise from breast-feeding even for COVID-19, there is some evidence that long-term immunity may be passed from mother to child through mother’s milk. Studies in animal models indicated that leukocytes from milk may infiltrate the tissues of intestinal tract, enter in mesenteric lymph nodes, and confer immunity to the recipient. It has also been shown that Most breast milk lymphocytes establish themselves in specific areas of the intestine termed Peyer’s patches (PPs). It appears that it is also possible for lymphocytes such as T and B cells have the ability to migrate from the blood to secondary lymphoid tissues such as spleen, lymph nodes, and Peyer’s patches, and then migrate back to the blood. While this is not a high-prevalence scenario, it could result in small amounts of long-lasting B mem that results in a level of future protection of the child resulting from breast-feeding. We know that oral immunization stimulate mucosal and systemic memory B cell development. It appears that in rodent models, perinatal antigen exposure via mother’s milk has been shown to prime the suckling animal’s immune responses in a manner so profound that the effects can still be detected two generations later.
Also See: Immune System Function
References



that the viral load of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
peaks within the first week of disease onset.1,2 Findings from Feb, 2020, indicated
that the clinical spectrum of this disease can…
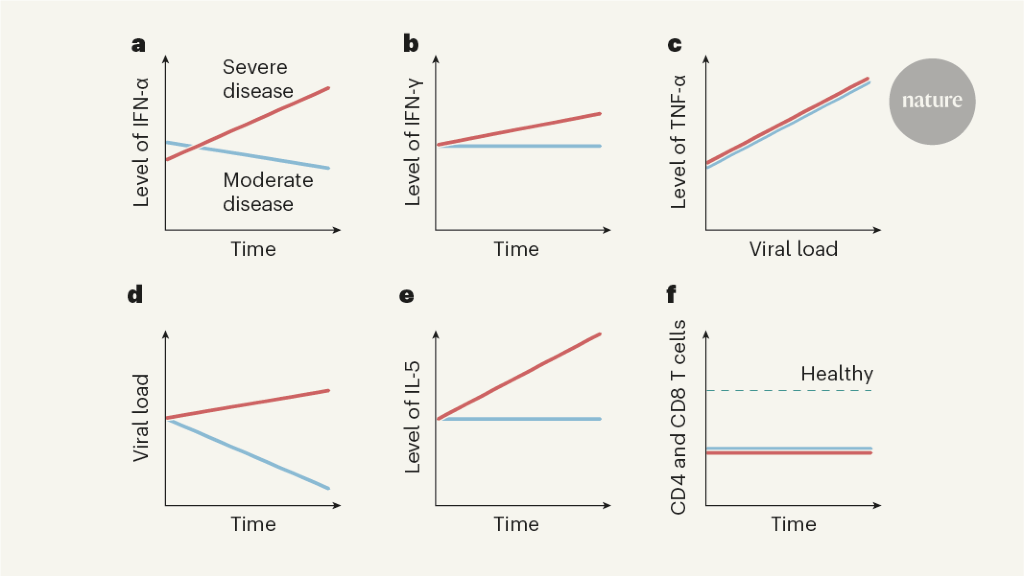




across the full spectrum of exposure, infection, and COVID-19 severity. They observe
that SARS-CoV-2 elicits broadly directed and functionally replete memory T cells that
may protect against recurrent episodes of se…





Another monkey study

Only New York showed more COVID-19 infections than Connecticut through early May, according to a report that also revealed the number of people infected by the virus is actually about six times more than the number reported by the state. The Centers for Disease Control and Prevention (CDC) studie…
“We performed a longitudinal analysis of antibody titers specific for viral antigens (vaccinia, measles, mumps, rubella, varicella–zoster virus, and Epstein–Barr virus) and nonreplicating antigens (tetanus and diphtheria) in 45 subjects for a period of up to 26 years. In addition, we measured antigen-specific memory B cells by means of limiting-dilution analysis, and we compared memory B-cell frequencies to their corresponding serum antibody levels.”
…
“These studies provide quantitative analysis of serologic memory for multiple antigens in subjects followed longitudinally over the course of more than one decade. In cases in which multiple exposures or repeated vaccinations were common, memory B-cell numbers did not correlate with antibody titers”

“there is no statistically significant linear correlation between the frequencies of circulating antigen-specific IgG-bearing memory B cells and the serum titers of antigen-specific IgG”

“In cases in which multiple exposures or repeated vaccinations were common, memory B-cell numbers did not correlate with antibody titers”

“After a disease outbreak, recovered individuals constitute a large immune population; however, their immunity is waning in the long term and they may become susceptible again. Meanwhile, their immunity can be boosted by repeated exposure to the pathogen, which is linked to the density of infected individuals present in the population.”





Neutralizing antibodies were detected even at the early stage of disease







Biodegradable vaccine delivery systems allow for prolonged antigen release and are a viable strategy to achieve single-dose administration, which is particularly desirable in the context of reducing number of repeated administrations required for long-term protection
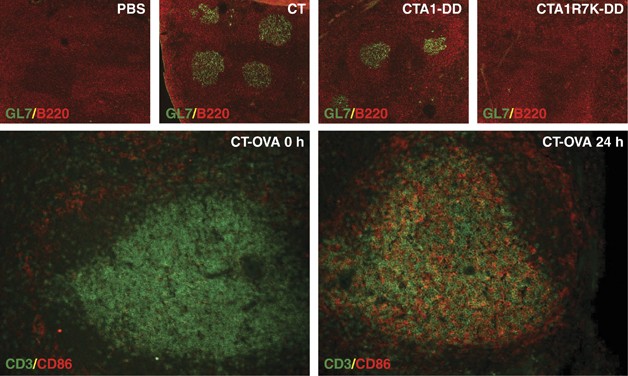


SARS-CoV-2 IgG assay is a chemiluminescent microparticle immunoassay




“In general, our elderly population has effective immunologic memory to things that they were exposed to when they were younger, but because this is a new virus, elderly people might have a difficult time generating an adaptive antiviral immune response.”
“if we are exposed to something multiple times, we generate robust and long-term immunity.”


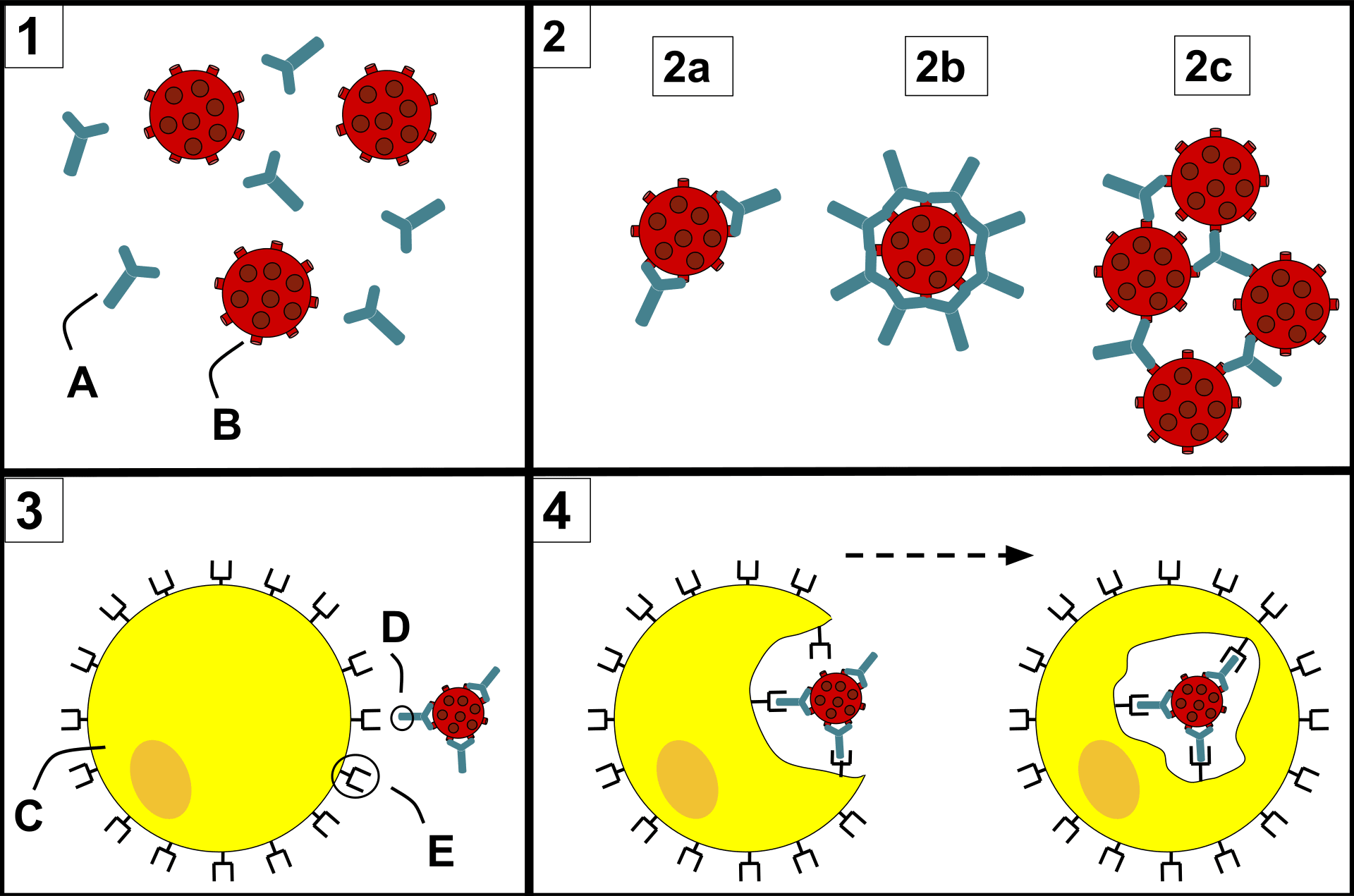
COVID-19 Vaccine Data

Manufacturing of mRNA Vaccines
The following mRNA manufacturing informational video discusses various purification techniques and challenges at about the 19 minute mark. Conceivably, the mRNA purification process leaves some undesirable mRNA elements within the resulting product. While most of these impurities would be inert and have no deleterious effects, it does raise the question about the possibility of extremely rare mRNA impurities that could result in undesirable protein synthesis by cellular ribosomes upon vaccination that then causes adverse events. Autoimmune related adverse events have been hypothesized. It seems plausible that certain unusual proteins could be related to the development of an autoimmune response. As the video states, more research and development effort to improve the purification process and a better understanding of the impact of impurities within mRNA synthesis process are desirable.

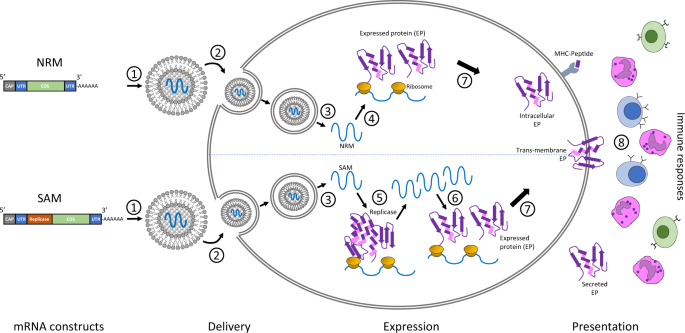


“At day 7, in animals vaccinated with 10 μg of mRNA-1273, inflammation was mild, and no viral RNA was detected (Figure 4A). However, at day 8, one animal in the 10-μg dose group had a single pneumocyte that was positive for viral antigen (Fig. S8). No substantial inflammation was observed in the lungs of nonhuman primates vaccinated with 100 μg of mRNA-1273, and neither viral RNA nor antigen was detected at day 7 or 8 after challenge (Figure 4A). In addition to the lung sections from these earlier time points, lung sections from animals that were killed at day 14 or 15 after challenge had no evidence of substantial inflammation, and neither viral RNA nor viral antigen was detected in any of the groups, including the control group”


significant immune responses in the majority of recipients after a single immunisation.

antibody responses. These results, together with the induction of both humoral and
cellular immune responses, support large-scale evaluation of this candidate vaccine
in an ongoing phase 3 programme.



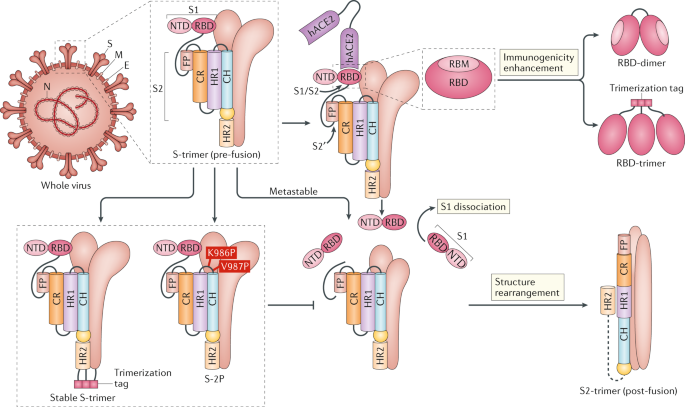







The doctor being interviewed in the video who received the vaccine stated that he participated in a trial and after second dose he had 24 hours of adverse symptoms including chills and muscle aches as a reaction to either the vehicle that delivers the mRNA or the protein produced by his body as a result of the vaccine. The reality is that his reaction is a normal part of an immune response. Immune responses may result in headaches, myalgia, and fever / chills. Symptoms are more often a result of the immune response, not the virus.
Regarding release of vaccines for use, there is a trade-off between agility and safety. It is interesting to revisit the idea of enrolling those at high-risk for a severe case during early trials. Would it have been better to run a parallel trial with specific groups having high comorbidities? Please contact us with your viewpoint and justification.

But you, and all the people who died, were prohibited by the government from taking it.

Pfizer First Dose Efficacy Was Incorrectly Calculated
The Pfizer vaccine phase 3 trial data indicated a first dose efficacy of 52.4%; however it appears this was incorrectly calculated. The calculation did not exclude the period where seroconversion was incomplete (the first 7 to 14 days). Efficacy calculations for the second dose excluded 7 days immediately after the second dose to allow for seroconversion delay. The first dose calculation used data from the time of the first dose to the time of the second dose and did not allow time for the vaccine to become effective. When using the data from Figure 3 of the Pfizer phase 3 trial and an appropriate exclusion period (the calculation is approximate due to missing data in the NEJM article), the efficacy can be calculated to over 90% (actually 93%). Sadly, the trial did not have a group that only received dose 1. From general immunology and many studies that show antibodies remaining for 3+ months, it can be deduced that dose 1 efficacy is likely to be greater than 90% and nearly equivalent to the dose 2 efficacy of 95% and remain durable for at least 3 months. Moderna’s first dose efficacy calculations were likely to be more correct at “an efficacy of 80.2 percent after the first dose”; however these may also not have allowed enough time for seroconversion, skewing the result downward from reality.
It is likely a large mistake if dose 2 is given at 3 weeks (4 weeks for Moderna) to the detriment of providing dose 1 much more widely to those at risk. The trial, being done at “warp speed” used a 3 week boost schedule to accelerate the trial. Many vaccine boosts (post trial) are typically done 6 months after prime with good results and there is currently little or no immunological data to suspect that a 3 or 6 month timing would not be appropriate. In fact, spacing out the prime and boost doses has the added effect of increasing the period of likely “sterilizing immunity”. It is also likely to increase the activation of additional naive B cells as antibody titers wane and do not neutralize the vaccine epitopes and preventing them from binding with B/T cells in the SLO germinal centers as part of a further binding affinity enhancement process which improves specificity and strength of future immune response. Providing a boost dose too early while unbound antibodies are highly prevalent can result in the antibodies binding to the boost vaccine epitopes and neutralizing them and preventing them from stimulating further immune response.
The 90% dose 1 efficacy recalculation was based on Pfizer trial data Figure 3 recalculations that added “Dose 2 to 7 days after dose 2” data to the “>=7 days after dose 2” data and also adding 3 more cases in the vaccinated group to approximate starting at “day 11 past dose 1”. This calculation is much more likely to reflect reality than the badly calculated 52.4% “After dose 1 to before dose 2” period which allowed zero time for the vaccine to take effect and complete seroconversion.


Vaccine Distribution And Administration




“The subsequent two weekly vaccine distributions could cover 7 to 10 million people a week, provided a second vaccine – from Moderna Inc – is authorized early in the second half of December, and Pfizer meets its distribution estimates, according to data provided by Department of Health and Human Services (HHS) and the companies.”
“In a statement Monday, Moderna said it plans to have 200 million doses for the U.S. by the second quarter, meeting its current commitment for the federal government” That calculates to about 7.7 million doses per week allocated to the US by Moderna alone.
For months it had been predicted that the vaccine would likely be available in December 2020. Numerous plans by vaccine manufacturers, shipping companies, and the US federal government were put in place to ensure earliest possible distribution – and they executed flawlessly. Within 72 hours of approval, the vaccines were nearly 100% distributed to points of care. Unfortunately, during week 1, most of the vaccine sat unused at point-of-care locations; less than 20% of available doses were administered and over 2 million US citizens who could have been protected, remained unprotected. Good execution is needed here. Given current knowledge about those at risk, there is already more than enough vaccine available on 12/21 to prevent the majority of deaths and serious hospitalizations. If deaths do not drop to less than 1/10th current rates before the end of January, it will be due to poor vaccination execution at the localities as the decisions about who to vaccinate and when is made at local levels. Vaccine sitting at points-of-care and not utilized is another indicator.
USA Data from CDC:
| Status Date | Total Doses Distributed | Total Doses Administered | Doses Unused | Percent Of Available Doses Actually Used | People That Got Dose 2 That Could Have Protected Others With Dose 1 |
|---|---|---|---|---|---|
| Dec 20, 2020 | 2838225 | 556208 | 2,282,017 | 19.6 | |
| Week of Dec 21, 2020 | 10738225 | ||||
| Dec 21 9AM ET | 4624325 | 614117 | 4,010,208 | 13.3 | |
| Dec 23 9AM ET | 9465725 | 1008025 | 8,457,700 | 10.6 | |
| Dec 26 9AM ET | 9547925 | 1944585 | 7,603,340 | 20.4 | |
| Dec 28 9AM ET | 11445175 | 2127143 | 9,318,032 | 18.6 | |
| Dec 30 9AM ET | 12409050 | 2794588 | 9,614,462 | 22.5 | |
| Jan 2 9AM ET | 13071925 | 4225756 | 8,846,169 | 32.3 | |
| Jan 4 9AM ET | 15418500 | 4563260 | 10,855,240 | 29.6 | |
| Jan 5 9AM ET | 17020575 | 4836469 | 12,184,106 | 28.4 | |
| Jan 6 9AM ET | 17288950 | 5306797 | 11,982,153 | 30.7 | |
| Jan 8 9AM ET | 22137350 | 6688231 | 15,449,119 | 30.2 | |
| Jan 11 9AM ET | 25480725 | 8987322 | 16,493,403 | 35.3 | |
| Jan 13 9AM ET | 29380125 | 10278462 | 19,101,663 | 35 | |
| Jan 15 6AM ET | 31161075 | 12279180 | 18,881,895 | 39.4 | |
| Jan 20 6AM ET | 35990150 | 16525281 | 19,464,869 | 45.9 | |
| Jan 22 6AM ET | 39892400 | 19107959 | 20,784,441 | 47.9 | |
| Jan 27 6AM ET | 47230950 | 24652634 | 22,578,316 | 52.2 | |
| Jan 29 6AM ET | 49216500 | 27884661 | 21,331,839 | 56.7 | |
| Feb 1 6AM ET | 49936450 | 32222402 | 17,714,048 | 64.5 | |
| Feb 3 6AM ET | 55943800 | 33878254 | 22,065,546 | 60.6 | |
| Feb 5 6AM ET | 58380300 | 36819212 | 21,561,088 | 63.1 | |
| Feb 8 6AM ET | 59307800 | 42417617 | 16,890,183 | 71.5 | 9,518,015 |
| Feb 10 6AM ET | 65972575 | 44769970 | 21,202,605 | 67.9 | 10,469,514 |
| Feb 13 6AM ET | 69883625 | 50641884 | 19,241,741 | 72.5 | 13,082,172 |
| Feb 17 6AM ET | 72423125 | 56281827 | 16,141,298 | 77.7 | 15,471,536 |
| Feb 23 6AM ET | 82114370 | 65032083 | 17,082,287 | 79.2 | 19,882,544 |
| Mar 2 6AM ET | 102353940 | 78631601 | 23,722,339 | 76.8 | 26,162,122 |
| Mar 9 6AM ET | 123232775 | 93692598 | 29,540,177 | 76 | 32,102,061 |
| Mar 16 6AM ET | 142918525 | 110737856 | 32,180,669 | 77.5 | 39,042,345 |
| Mar 23 6AM ET | 164300795 | 128217029 | 36,083,766 | 78 | 45,533,962 |
| Mar 31 6AM ET | 195581725 | 150273292 | 45,308,433 | 76.8 | 54,607,041 |
| Apr 7 6AM ET | 225294435 | 171476655 | 53,817,780 | 76.1 | 64,422,618 |
| Apr 15 6AM ET | 255400665 | 198317040 | 57,083,625 | 77.6 | 78,498,290 |
| Apr 21 6AM ET | 277938875 | 215951909 | 61,986,966 | 77.7 | 87,592,646 |
| Apr 28 6AM ET | 301857885 | 234639414 | 67,218,471 | 77.7 | 98,044,421 |
| May 13 6AM ET | 339165445 | 266596486 | 72,568,959 | 78.6 | 118,987,308 |
| May 24 6AM ET | 357250475 | 286890900 | 70,359,575 | 80.3 | 130,615,797 |